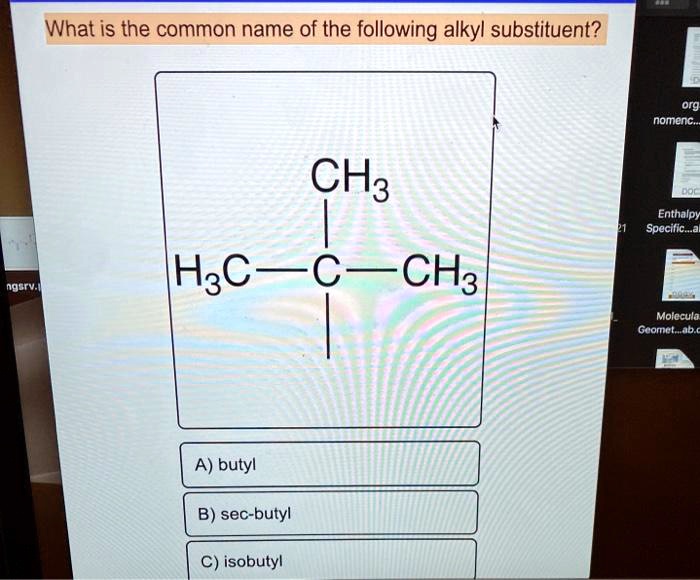
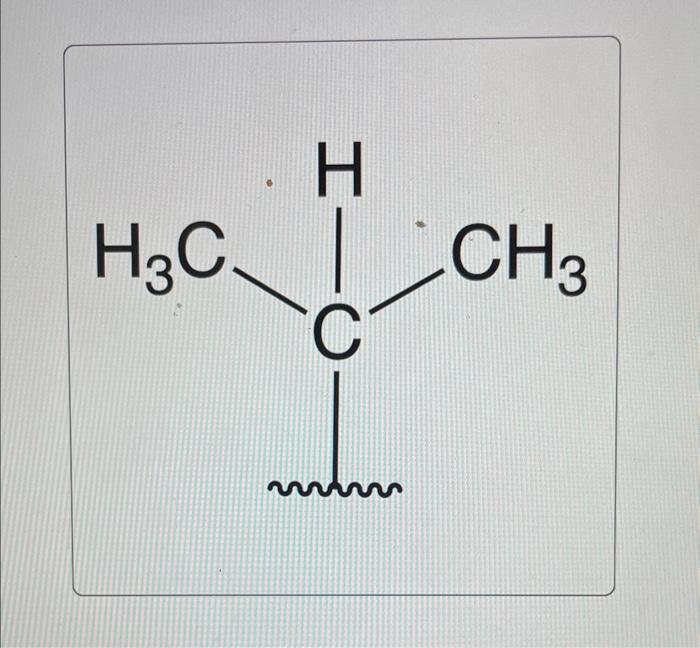
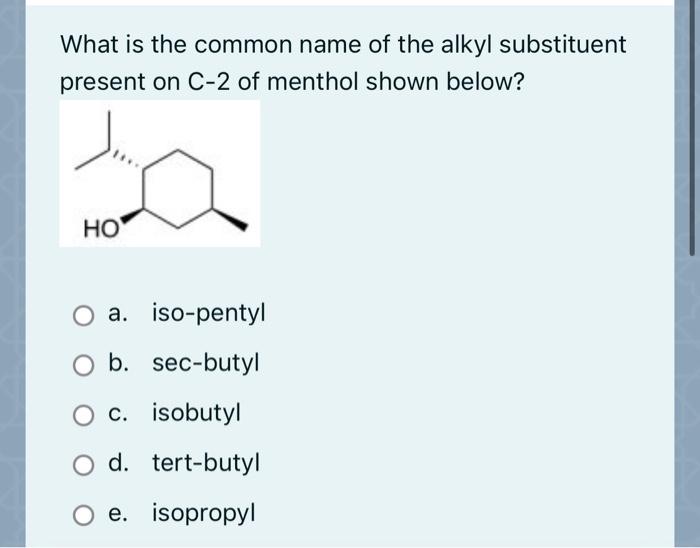
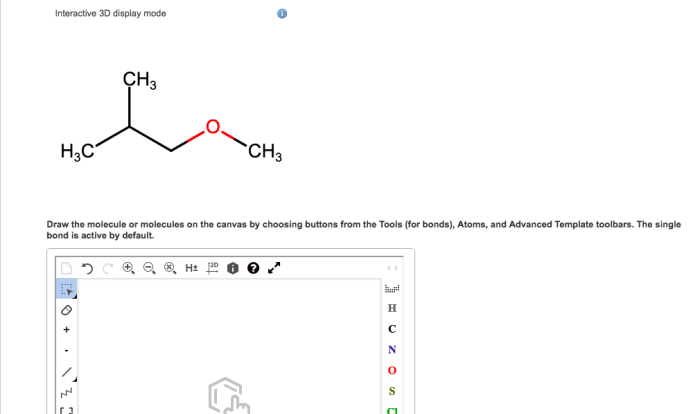
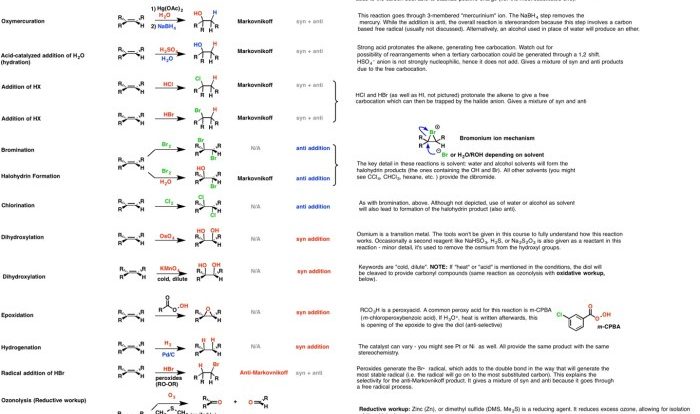
Choose the correct common name of the following alkyl substituent. – Nomenclature in organic chemistry is a systematic approach to naming organic compounds, ensuring clear and unambiguous communication among chemists. This article focuses on alkyl substituents, which are hydrocarbon groups attached to a parent structure. Understanding the IUPAC rules and common names for alkyl substituents is crucial for effective chemical communication and accurate representation of molecular structures.
The IUPAC (International Union of Pure and Applied Chemistry) guidelines provide a standardized framework for naming alkyl substituents based on their structure and size. These rules ensure consistency and minimize confusion in chemical nomenclature. Additionally, common names, often derived from historical or practical usage, are widely employed for convenience and familiarity.
This article explores both IUPAC and common names of alkyl substituents, providing a comprehensive guide for choosing the correct nomenclature in various contexts.
Nomenclature of Alkyl Substituents: Choose The Correct Common Name Of The Following Alkyl Substituent.
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming alkyl substituents, which are groups of atoms derived from alkanes by removing one hydrogen atom. These rules ensure consistency and clarity in organic chemistry.
To name an alkyl substituent, follow these steps:
- Identify the parent alkane by removing the hydrogen atom.
- Change the
- ane suffix to
- yl.
- Add a prefix to indicate the number of carbon atoms in the parent alkane.
For example, the alkyl substituent derived from propane (CH 3CH 2CH 3) is named propyl (CH 3CH 2CH 2-).
Common Names of Alkyl Substituents, Choose the correct common name of the following alkyl substituent.
In addition to IUPAC names, alkyl substituents also have common names. These names are often used in informal settings or when the structure of the substituent is clear from context.
| Molecular Formula | Structural Formula | Common Name |
|---|---|---|
| CH3– | H3C- | Methyl |
| CH3CH2– | H3C-CH2– | Ethyl |
| CH3CH2CH2– | H3C-CH2-CH2– | Propyl |
| CH3CH2CH2CH2– | H3C-CH2-CH2-CH2– | Butyl |
Choosing the Correct Common Name
When choosing the correct common name for an alkyl substituent, consider the following criteria:
- The length of the carbon chain.
- The presence of any functional groups.
- The context in which the name is being used.
For example, the common name for the alkyl substituent CH 3CH 2CH(CH 3)- is isopropyl, because it is a three-carbon chain with a methyl group attached to the middle carbon.
Exceptions to the Rules
There are a few exceptions to the IUPAC rules for naming alkyl substituents. These exceptions are typically due to historical usage or the desire to avoid confusion.
- The alkyl substituent derived from benzene (C 6H 6) is named phenyl, not benzyl.
- The alkyl substituent derived from toluene (C 7H 8) is named benzyl, not tolyl.
It is important to be aware of these exceptions to ensure accurate and consistent communication in organic chemistry.
Top FAQs
What is the purpose of naming alkyl substituents?
Naming alkyl substituents allows chemists to clearly identify and describe the hydrocarbon groups attached to a parent structure, facilitating precise communication and accurate representation of molecular structures.
Why is it important to use correct nomenclature for alkyl substituents?
Correct nomenclature ensures consistency and minimizes confusion in chemical communication, enabling scientists to accurately convey the identity and structure of organic compounds.
What are some common exceptions to the IUPAC rules for naming alkyl substituents?
Exceptions to the IUPAC rules exist for certain alkyl substituents, such as the tert-butyl group, which retains its common name due to historical usage and familiarity.