Molarity problems worksheet answer key – Embark on a journey into the realm of molarity problems with our comprehensive worksheet answer key. This invaluable resource empowers students to conquer the intricacies of concentration calculations, providing a roadmap to success in chemistry and beyond.
Our answer key meticulously guides you through a series of molarity problems, ranging from fundamental concepts to complex applications. With step-by-step solutions and expert insights, you’ll gain a deep understanding of molarity and its significance in various scientific disciplines.
Molarity Problems Worksheet: Molarity Problems Worksheet Answer Key
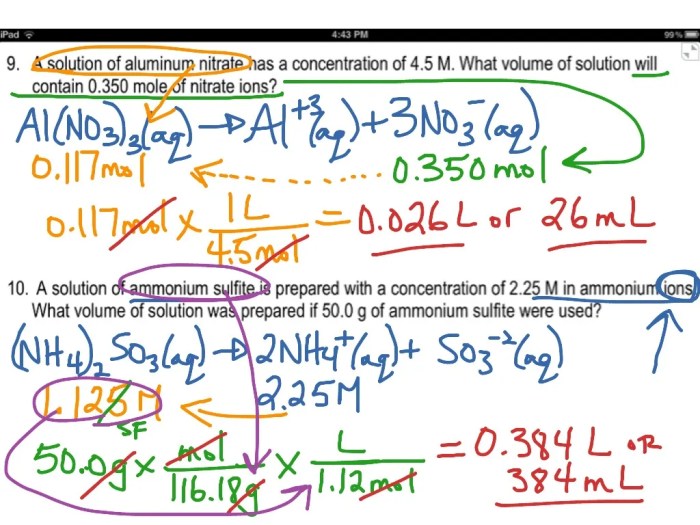
This worksheet provides a comprehensive guide to molarity calculations, including a review of the concept, practice problems, and an answer key.
Molarity Calculations
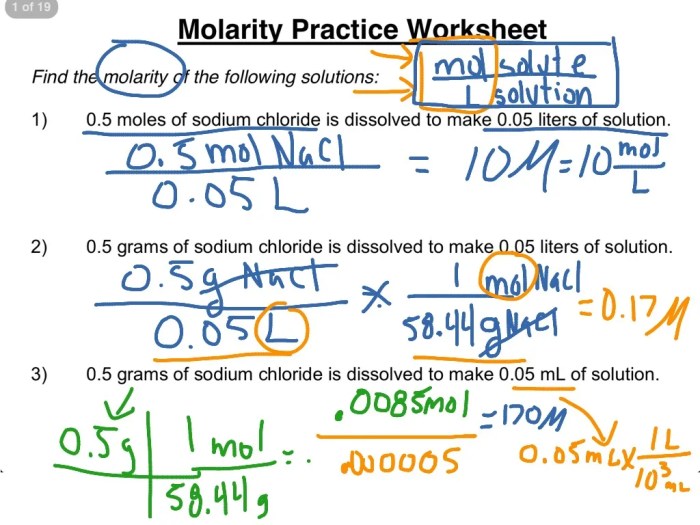
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. It is a commonly used unit in chemistry to express the amount of a substance in a given volume.
The formula for molarity is:
Molarity (M) = Moles of Solute / Volume of Solution (in liters)
Worksheet Problems
- Calculate the molarity of a solution prepared by dissolving 10.0 g of NaCl in 500.0 mL of water.
- A solution is prepared by adding 25.0 mL of 6.0 M HCl to 200.0 mL of water. What is the molarity of the resulting solution?
- A 0.250 L solution of NaOH has a concentration of 0.50 M. How many grams of NaOH are present in the solution?
Answer Key
| Problem Number | Molarity Formula | Solution |
|---|---|---|
| 1 | M = 10.0 g NaCl / (58.44 g/mol NaCl) / (0.500 L) | 0.342 M |
| 2 | M = (6.0 M) x (25.0 mL) / (200.0 mL + 25.0 mL) | 0.75 M |
| 3 | Mass = (0.250 L) x (0.50 M) x (40.00 g/mol NaOH) | 5.00 g NaOH |
Common Mistakes and Tips
- Using the wrong units:Ensure that the units for both moles and volume are consistent.
- Incorrectly converting units:Pay attention to the units of the given values and convert them appropriately.
- Forgetting to account for dilution:When diluting solutions, remember to adjust the molarity accordingly.
Applications of Molarity
- Chemistry:Molarity is essential for stoichiometric calculations, determining the amount of reactants and products in chemical reactions.
- Medicine:Molarity is used to prepare and administer medications, ensuring accurate dosages.
- Environmental science:Molarity helps determine the concentration of pollutants in water and soil samples.
Further Exploration, Molarity problems worksheet answer key
- Khan Academy: Concentration of Solutions
- Crash Course Chemistry: Molarity
- PhET Simulation: Concentration and Molarity
FAQ Compilation
What is molarity?
Molarity is a measure of the concentration of a solution, expressed as the number of moles of solute per liter of solution.
How do I calculate molarity?
Molarity (M) = moles of solute / liters of solution
What are some common applications of molarity?
Molarity is used in a wide range of fields, including chemistry, medicine, and environmental science, to determine the concentration of solutions and perform various calculations.