Introduction to Balancing Equations Worksheet Key Answer Key: A Comprehensive Guide to Mastering Chemical Reactions
Balancing chemical equations is a fundamental skill in chemistry, ensuring the conservation of mass and providing a deeper understanding of chemical reactions. This worksheet key offers a step-by-step guide, empowering learners to confidently navigate the intricacies of balancing equations and unlock the secrets of chemical transformations.
Introduction to Balancing Equations Worksheet Key Answer Key

Balancing equations is a crucial step in understanding and predicting the outcome of chemical reactions. This worksheet provides a comprehensive guide to the fundamental principles and key steps involved in balancing equations, ensuring the conservation of mass and the accuracy of chemical representations.
Understanding the Basics of Balancing Equations
Chemical equations are symbolic representations of chemical reactions, showing the reactants and products involved. Balancing equations ensures that the number of atoms of each element on the reactants’ side matches the number on the products’ side. This adherence to the law of conservation of mass is essential for accurate chemical calculations and predictions.
Methods for Balancing Equations
There are several methods for balancing equations, including the half-reaction method, oxidation-reduction method, and matrix method. Each method follows specific steps and principles to adjust the coefficients in front of each chemical formula, ensuring the equation is balanced.
Applications of Balancing Equations
Balancing equations has wide-ranging applications in chemistry, engineering, and medicine. It allows chemists to predict the products and quantities of reactants in chemical reactions, design experiments, and analyze reaction mechanisms. In engineering, balancing equations is crucial for process design and optimization, while in medicine, it aids in understanding metabolic pathways and drug interactions.
Practice and Review, Introduction to balancing equations worksheet key answer key
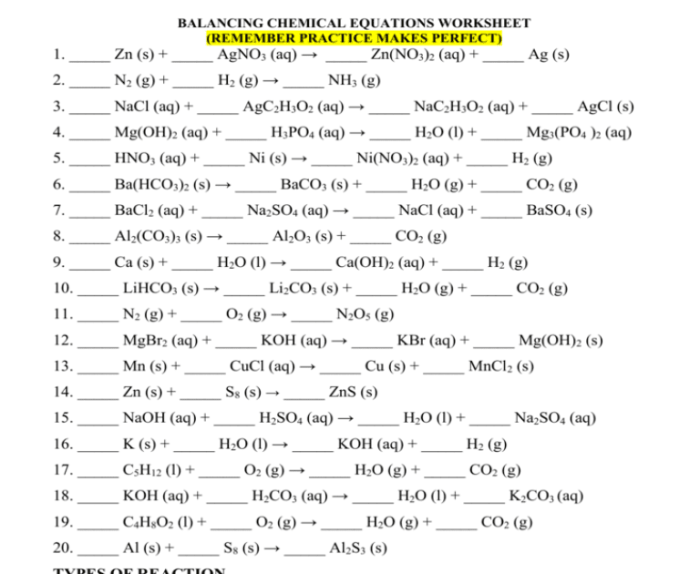
Practice problems and exercises are essential for reinforcing the understanding of balancing equations. This worksheet provides a series of problems with varying difficulty levels, allowing learners to test their skills and gain proficiency in this fundamental chemical concept.
Question & Answer Hub: Introduction To Balancing Equations Worksheet Key Answer Key
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of the same element on the products’ side, upholding the law of conservation of mass.
What are the key steps involved in balancing equations?
Balancing equations involves adjusting the stoichiometric coefficients in front of each chemical formula until the number of atoms of each element is identical on both sides of the equation.
How can I practice balancing equations effectively?
Regular practice is crucial for mastering balancing equations. Utilize the practice problems and exercises provided in this worksheet key, along with additional resources, to reinforce your understanding.