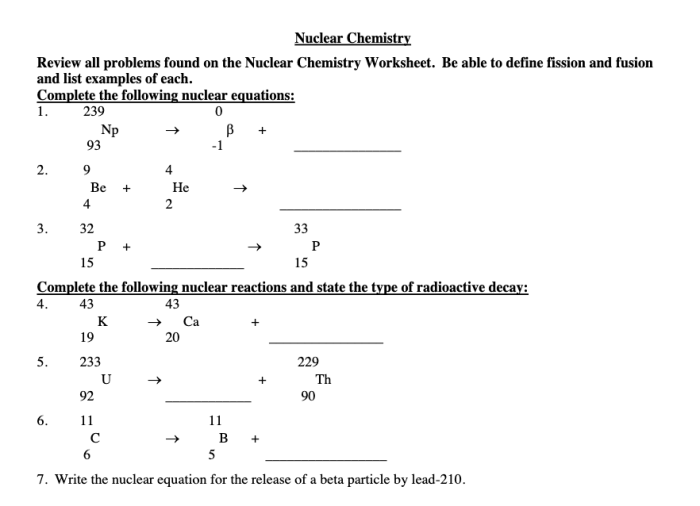
Nuclear chemistry review worksheet answer key – Embark on a journey into the fascinating realm of nuclear chemistry with our comprehensive answer key for review worksheets. This guide delves into the fundamental principles, reactions, and applications of nuclear chemistry, providing a profound understanding of this captivating field.
From the structure of atoms to the intricacies of nuclear reactions and the practical applications of radioactivity, this answer key unveils the complexities of nuclear chemistry with clarity and precision.
Nuclear Chemistry Basics
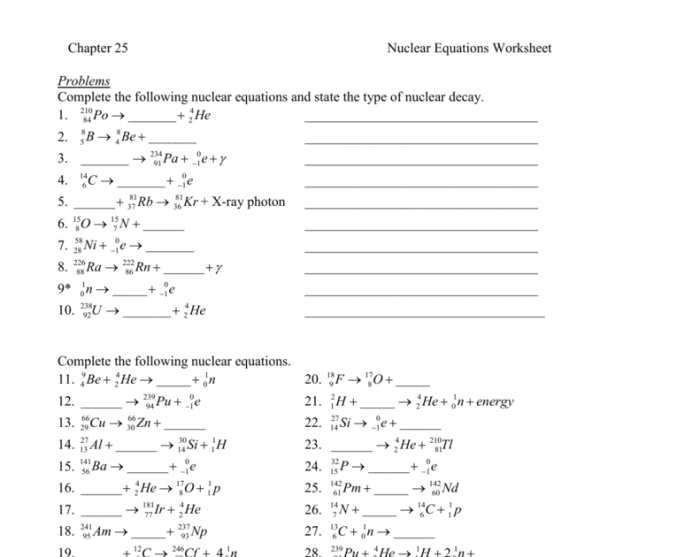
Nuclear chemistry is the study of the structure, properties, and reactions of atomic nuclei. It is a branch of chemistry that deals with the interactions of the fundamental particles that make up the nucleus, including protons, neutrons, and electrons.
Structure of an Atom
The atom is the basic unit of matter. It is composed of a nucleus, which is located at the center of the atom and contains the protons and neutrons, and electrons, which orbit the nucleus.
The nucleus is very small compared to the rest of the atom, but it contains most of the atom’s mass. Protons are positively charged particles, while neutrons are neutral particles. The number of protons in an atom’s nucleus determines its atomic number, which is unique for each element.
Electrons are negatively charged particles that orbit the nucleus in shells. The number of electrons in an atom is equal to the number of protons, so atoms are electrically neutral.
Atomic Number and Mass Number, Nuclear chemistry review worksheet answer key
The atomic number of an atom is the number of protons in its nucleus. The mass number of an atom is the total number of protons and neutrons in its nucleus.
The mass number of an atom is approximately equal to its atomic weight, which is the average mass of all the isotopes of that element.
Isotopes
Isotopes are atoms of the same element that have the same atomic number but different mass numbers. This means that isotopes have the same number of protons but different numbers of neutrons.
Isotopes are used in a variety of applications, including medicine, industry, and research.
FAQ Insights: Nuclear Chemistry Review Worksheet Answer Key
What is the difference between atomic number and mass number?
Atomic number represents the number of protons in an atom, defining its elemental identity, while mass number represents the total number of protons and neutrons in the atom’s nucleus.
Explain the concept of isotopes and their applications.
Isotopes are atoms of the same element with varying numbers of neutrons. They find applications in medicine (radioactive isotopes for imaging and therapy), industry (tracers for material analysis), and research (dating techniques).