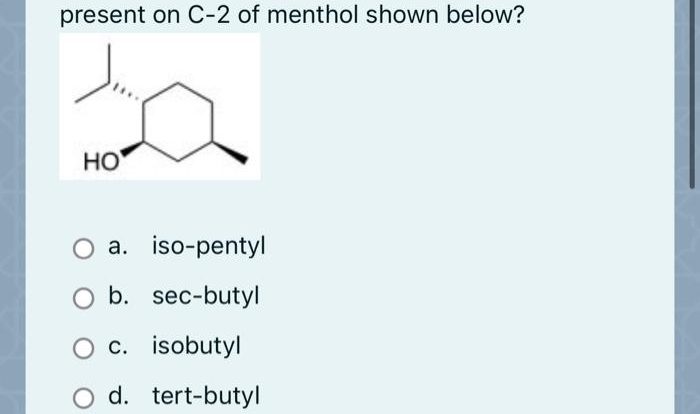
Welcome to the comprehensive alkene addition reactions cheat sheet, your ultimate guide to understanding and mastering these fundamental reactions in organic chemistry. Dive into the world of alkene addition reactions and gain a deep understanding of their mechanisms, regioselectivity, stereoselectivity, and diverse applications.
As you embark on this journey, you’ll explore the intricacies of Markovnikov’s rule, unravel the mechanisms of electrophilic and radical alkene addition reactions, and discover the practical applications of these reactions in organic synthesis and industry.
Alkene Addition Reactions
Alkene addition reactions are a type of organic chemical reaction in which two or more atoms or groups of atoms are added to a carbon-carbon double bond. These reactions are typically catalyzed by a Lewis acid, such as H +or BF 3.
Types of Alkene Addition Reactions
There are three main types of alkene addition reactions:
- Electrophilic addition reactions
- Nucleophilic addition reactions
- Radical addition reactions
Regioselectivity and Stereoselectivity
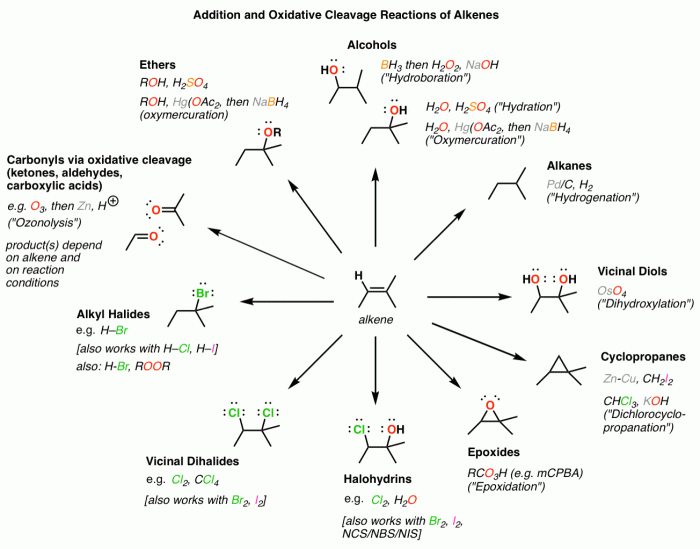
In alkene addition reactions, regioselectivity and stereoselectivity are two important factors that determine the orientation and configuration of the newly formed bonds.
Regioselectivity
Regioselectivity refers to the preference for one regioisomer over another. In alkene addition reactions, the regioselectivity is determined by the stability of the carbocation intermediate formed during the reaction. The more stable carbocation intermediate will lead to the major product.
Stereoselectivity
Stereoselectivity refers to the preference for one stereoisomer over another. In alkene addition reactions, the stereoselectivity is determined by the orientation of the attacking nucleophile relative to the double bond. The orientation of the nucleophile can be controlled by the steric and electronic effects of the substituents on the alkene.
Markovnikov’s Rule
Markovnikov’s rule is an empirical observation that governs the regioselectivity of electrophilic addition reactions of unsymmetrical alkenes. It states that the positive portion of the electrophile (usually a hydrogen atom or a carbocation) adds to the carbon atom of the double bond that has the most hydrogen atoms.
Markovnikov’s rule can be explained by the stability of the intermediate carbocation. The more substituted the carbocation, the more stable it is. This is because the alkyl groups donate electrons to the carbocation, which helps to stabilize it.
Exceptions to Markovnikov’s Rule
There are a number of exceptions to Markovnikov’s rule. These exceptions occur when the electrophile is very reactive or when the alkene is very hindered. In these cases, the positive portion of the electrophile may add to the carbon atom of the double bond that has the fewest hydrogen atoms.
Some of the most common exceptions to Markovnikov’s rule include:
- Addition of hydrogen bromide to 1-butene
- Addition of hydrogen iodide to 2-methylpropene
- Addition of sulfuric acid to 1-methylcyclohexene
Mechanisms of Alkene Addition Reactions: Alkene Addition Reactions Cheat Sheet
Alkene addition reactions are a class of organic reactions in which an electrophile or a radical adds to a double bond, resulting in the formation of a new single bond and a new carbon-carbon bond.
The mechanisms of alkene addition reactions can be classified into two main types: electrophilic addition and radical addition.
Electrophilic Alkene Addition Reactions
Electrophilic alkene addition reactions proceed through a two-step mechanism:
- The electrophile attacks the double bond, forming a new bond between the electrophile and one of the carbon atoms in the double bond.
- The resulting carbocation is then attacked by a nucleophile, which completes the addition reaction.
Electrophilic alkene addition reactions are typically catalyzed by acids, which activate the electrophile and make it more reactive towards the double bond.
Radical Alkene Addition Reactions
Radical alkene addition reactions proceed through a three-step mechanism:
- A radical initiator generates a radical, which then attacks the double bond, forming a new bond between the radical and one of the carbon atoms in the double bond.
- The resulting radical intermediate reacts with another radical, forming a new carbon-carbon bond.
- The radical intermediate is then terminated by a radical scavenger, which completes the addition reaction.
Radical alkene addition reactions are typically initiated by heat, light, or peroxides.
Applications of Alkene Addition Reactions

Alkene addition reactions are widely used in organic synthesis for the preparation of various functionalized compounds. They are also employed in numerous industrial applications.
Organic Synthesis
- Hydrohalogenation:Addition of hydrogen halides (HX) to alkenes yields alkyl halides, which are versatile intermediates for further reactions.
- Hydration:Addition of water to alkenes in the presence of acid catalysts forms alcohols.
- Halogenation:Addition of halogens (X 2) to alkenes yields vicinal dihalides, which are useful for the synthesis of alkenes with different substitution patterns.
- Hydroboration-Oxidation:This reaction sequence adds boron and then oxygen to alkenes, yielding alcohols with anti-Markovnikov regioselectivity.
- Epoxidation:Addition of peroxyacids (RCO 3H) to alkenes forms epoxides, which are reactive intermediates for various transformations.
Industrial Applications, Alkene addition reactions cheat sheet
- Polyethylene and Polypropylene Production:Alkene addition reactions are used in the polymerization of ethylene and propylene to produce polyethylene and polypropylene, respectively, which are widely used plastics.
- Synthetic Rubber Production:Alkene addition reactions are employed in the synthesis of synthetic rubbers, such as styrene-butadiene rubber (SBR) and polyisoprene, which are used in tires and other rubber products.
- Detergent Production:Alkene addition reactions are used in the production of alkylbenzene sulfonates (ABS), which are anionic surfactants commonly used in detergents.
Table of Common Alkene Addition Reactions
This table provides a concise summary of common alkene addition reactions, including the reactants, products, reaction conditions, regioselectivity, and stereoselectivity of each reaction.
The regioselectivity of an alkene addition reaction refers to the preference for the addition of the electrophile to one of the two carbon atoms of the double bond. The stereoselectivity of an alkene addition reaction refers to the preference for the formation of one stereoisomer over the other.
Table of Common Alkene Addition Reactions
| Reaction Type | Reactants | Products | Reaction Conditions | Regioselectivity | Stereoselectivity |
|---|---|---|---|---|---|
| Hydrohalogenation | Alkene + HX | Alkyl halide | Polar aprotic solvent | Markovnikov | Anti |
| Oxymercuration-Demercuration | Alkene + Hg(OAc)2 + H2O | Alcohol | Water | Markovnikov | Syn |
| Hydroboration-Oxidation | Alkene + BH3 + H2O2 + NaOH | Alcohol | THF | Anti-Markovnikov | Syn |
| Epoxidation | Alkene + m-CPBA | Epoxide | Dichloromethane | – | – |
| Diels-Alder Reaction | Diene + Dienophile | Cyclohexene | Heat | – | – |
Practice Problems

To enhance your understanding of alkene addition reactions, let’s delve into a series of practice problems.
The following exercises will provide an opportunity to apply the concepts and mechanisms discussed earlier. Attempting these problems will reinforce your knowledge and prepare you for more complex applications of alkene addition reactions.
Practice Set
1. Predict the major product(s) of the following alkene addition reactions
CH3CH=CH2 + HBr
(CH3)2C=CH2 + HCl
CH2=CHCH2CH3 + H2O (acid-catalyzed)
2. Determine the regioselectivity of the following reactions based on Markovnikov’s rule
CH3CH=CH2 + H2O (acid-catalyzed)
(CH3)2C=CH2 + HBr
- CH2=CHCH2CH3 + HCl
- Propose a plausible mechanism for the acid-catalyzed addition of water to an alkene. Include the key intermediates and steps involved.
- Explain how stereochemistry influences the outcome of alkene addition reactions. Provide examples to illustrate your explanation.
- Design a synthetic route to convert an alkene into a specific alcohol or ether using an alkene addition reaction. Artikel the necessary steps and reagents involved.
Query Resolution
What is the Markovnikov’s rule?
Markovnikov’s rule states that in the addition of a protic acid to an unsymmetrical alkene, the proton adds to the carbon-carbon double bond in a way that results in the formation of the more substituted carbocation.
What is the difference between regioselectivity and stereoselectivity?
Regioselectivity refers to the preference for one regioisomer over another, while stereoselectivity refers to the preference for one stereoisomer over another.
What are the applications of alkene addition reactions?
Alkene addition reactions are widely used in organic synthesis for the production of a variety of compounds, including alcohols, ethers, and alkenes.