Embark on a captivating journey with our flame test lab answers pdf, where the enigmatic dance of elements unfolds before your eyes. Prepare to be mesmerized as we unravel the secrets hidden within the vibrant hues of flames, revealing the identities of unknown substances with scientific precision.
Delve into the fascinating world of flame tests, where the interplay of heat and chemical composition paints a colorful canvas of elemental signatures. Discover the fundamental principles that govern these mesmerizing reactions, and equip yourself with the knowledge to interpret the chromatic language of flames.
Flame Test Basics
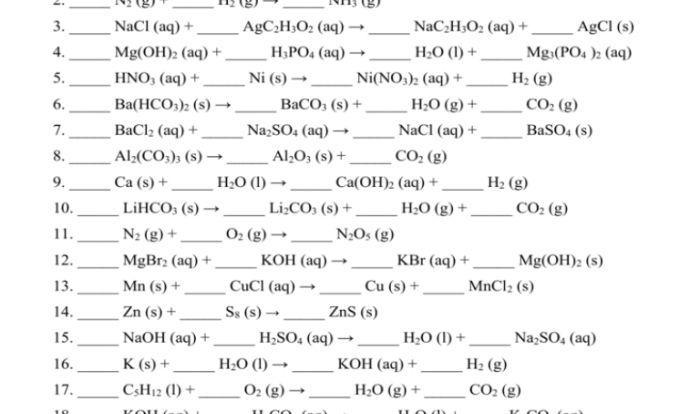
A flame test is a simple analytical technique used to identify the presence of certain elements in a sample. It involves heating a sample in a flame and observing the color of the flame produced.
The color of the flame is determined by the electronic structure of the element being tested. When an element is heated, its electrons absorb energy and move to higher energy levels. When the electrons return to their original energy levels, they release energy in the form of light.
The wavelength of the light emitted corresponds to the energy difference between the two energy levels.
Types of Flame Tests, Flame test lab answers pdf
There are two main types of flame tests: emission flame tests and absorption flame tests.
- Emission flame testsare used to identify the presence of elements that emit light when heated. The sample is placed in a flame, and the color of the flame is observed.
- Absorption flame testsare used to identify the presence of elements that absorb light at specific wavelengths. The sample is placed in a flame, and the flame is passed through a spectrometer. The spectrometer measures the amount of light absorbed at each wavelength, and this information can be used to identify the elements present in the sample.
Applications of Flame Tests
Flame tests are used in a variety of applications, including:
- Qualitative analysis: Flame tests can be used to identify the presence of certain elements in a sample. This information can be used to determine the composition of a sample or to identify unknown substances.
- Quantitative analysis: Flame tests can be used to determine the concentration of an element in a sample. This information can be used to control the concentration of an element in a product or to monitor the progress of a reaction.
- Educational purposes: Flame tests are often used in educational settings to demonstrate the principles of spectroscopy and to teach students about the different elements.
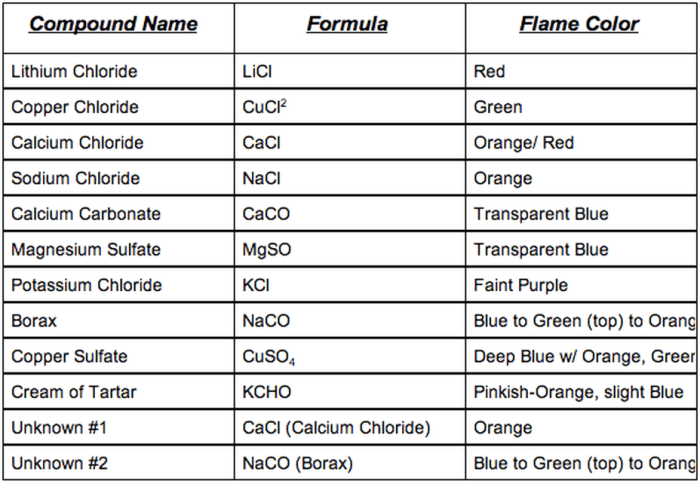
Flame Test Colors and Corresponding Elements
The following table summarizes the flame test colors and the corresponding elements:
| Flame Color | Element |
|---|---|
| Red | Lithium |
| Orange | Calcium |
| Yellow | Sodium |
| Green | Barium |
| Blue | Copper |
| Violet | Potassium |
Flame Test Procedure
The flame test is a simple and inexpensive analytical technique used to identify the presence of certain elements in a sample. It is based on the principle that when a compound is heated in a flame, the electrons in the compound’s atoms absorb energy and become excited.
When the electrons return to their ground state, they release energy in the form of light. The wavelength of the emitted light is characteristic of the element that is present in the compound.
The flame test procedure is as follows:
Materials
- Sample to be tested
- Platinum wire or nichrome wire loop
- Bunsen burner
- Safety goggles
- Gloves
Safety Precautions
- Wear safety goggles and gloves when performing the flame test.
- Do not point the flame towards yourself or others.
- Keep the sample away from the flame until it is ready to be tested.
- Do not touch the platinum wire or nichrome wire loop while it is hot.
Procedure
- Clean the platinum wire or nichrome wire loop by dipping it in hydrochloric acid and then heating it in the flame until it glows red.
- Dip the platinum wire or nichrome wire loop into the sample to be tested.
- Hold the platinum wire or nichrome wire loop in the flame.
- Observe the color of the flame.
- Compare the color of the flame to a known color chart to identify the element that is present in the sample.
Flame Test Observations and Interpretation
Flame tests are simple yet effective methods for determining the elemental composition of a substance. By observing the characteristic color changes produced when a substance is heated in a flame, we can identify and distinguish between different elements.
The color of the flame is determined by the electronic structure of the element. When an element is heated, its electrons absorb energy and become excited. As the electrons return to their ground state, they release energy in the form of light, producing the characteristic flame color.
If you’re looking for answers to your flame test lab, you’ve come to the right place. Our comprehensive guide will help you understand the basics of flame tests and how to interpret your results. To enhance your learning, we recommend exploring our article on trapezoids and kites 6 6 . This will provide you with a deeper understanding of geometric shapes, which can be applied to various scientific concepts, including flame tests.
By combining knowledge from both topics, you’ll gain a well-rounded understanding of scientific principles.
Distinguishing Elements Based on Flame Colors
Each element emits a unique flame color, allowing us to identify and distinguish between them. Some common elements and their characteristic flame colors include:
- Sodium (Na): Intense yellow
- Potassium (K): Pale violet
- Calcium (Ca): Brick red
- Copper (Cu): Green
- Strontium (Sr): Crimson red
By comparing the flame color produced by an unknown substance to these known colors, we can make a qualitative determination of the elements present.
Applications of Flame Tests
Flame tests find practical applications in various fields, including chemistry, forensic science, and geology.
In qualitative analysis, flame tests are employed to identify the presence of specific elements in a sample. By observing the characteristic color of the flame produced when a sample is heated in a flame, analysts can determine the presence of elements such as sodium, potassium, calcium, and copper.
Elemental Identification
- In forensic science, flame tests are used to analyze trace evidence, such as gunshot residue, to identify the presence of elements like barium and lead, which are commonly found in ammunition.
- In geology, flame tests are used to identify the elemental composition of minerals and rocks, aiding in mineral exploration and classification.
- In the semiconductor industry, flame tests are employed to detect impurities in silicon wafers used in the production of electronic devices.
Troubleshooting Flame Tests
Flame tests are generally straightforward, but certain errors and challenges can arise. Understanding these issues and implementing proper troubleshooting techniques is crucial for accurate results.
Common Errors and Challenges
* Faint or Inaccurate Flame Colors:This can occur due to impurities in the sample, insufficient heating, or contamination of the burner.
Interfering Ions
The presence of certain ions, such as sodium or potassium, can mask the flame color of other elements.
Improper Calibration
Incorrect calibration of the flame test apparatus can lead to inaccurate results.
Troubleshooting Tips
* Ensure Sample Purity:Use high-purity samples or purify them before testing.
Heat the Sample Thoroughly
Hold the sample in the hottest part of the flame for a sufficient time.
Clean the Burner Regularly
Remove any buildup or contamination from the burner tip to prevent cross-contamination.
Use a Spectrometer
If possible, use a spectrometer to obtain more accurate and precise flame test results.
Calibrate the Apparatus
Regularly calibrate the flame test apparatus using known standards to ensure accuracy.
Importance of Proper Technique
Proper technique is essential for accurate flame test results. This includes using clean glassware, holding the sample correctly, and observing the flame color carefully. Consistent technique helps minimize errors and ensures reliable results.
Additional Resources
Delve deeper into the fascinating world of flame tests with these valuable resources:
Recommended Books and Articles
- Flame Tests: A Practical Guideby John Emsley
- Chemical Demonstrations: A Handbook for Teachers of Chemistryby Bassam Z. Shakhashiri
- “Flame Tests: A Simple Way to Identify Elements” by Science Buddies
Flame Test Kits and Materials
Online Resources and Forums
- Science Forums Chemistry Forum
- Reddit Chemistry Subreddit
- Michigan State University Flame Tests Resource
Q&A: Flame Test Lab Answers Pdf
What is the purpose of a flame test?
Flame tests are used to determine the elemental composition of a substance by observing the color of the flame produced when the substance is heated.
How do I interpret the results of a flame test?
Each element produces a characteristic flame color. By comparing the color of the flame to a known color chart, you can identify the elements present in the substance.
What are some common applications of flame tests?
Flame tests are used in various fields, including qualitative analysis, forensic science, and geology, to identify elements in unknown substances.